


NOBELFÖRSAMLINGEN KAROLINSKA INSTITUTET
THE NOBEL ASSEMBLY AT THE KAROLINSKA INSTITUTE
The Nobel Assembly at the Karolinska Institute has today decided to award the Nobel Prize in Physiology or Medicine for 1991 jointly to
Erwin Neher and Bert Sakmann
for their discoveries concerning “the function of single ion channels in cells”.
Each living cell is surrounded by a membrane which separates the world within the cell from its exterior. In this membrane there are channels, through which the cell communicates with its surroundings. These channels consist of single molecules or complexes of molecules and have the ability to allow passage of charged atoms, that is ions. The regulation of ion channels influences the life of the cell and its functions under normal and pathological conditions. The Nobel Prize in Physiology or Medicine for 1991 is awarded for the discoveries of the function of ion channels. The two German cell physiologists Erwin Neher and Bert Sakmann have together developed a technique that allows the registration of the incredibly small electrical currents (amounting to a picoampere – 10-12A) that passes through a single ion channel. The technique is unique in that it records how a single channel molecule alters its shape and in that way controls the flow of current within a time frame of a few millionths of a second.
Neher and Sakmann conclusively established with their technique that ion channels do exist and how they function. They have demonstrated what happens during the opening or closure of an ion channel with a diameter corresponding to that of a single sodium or chloride ion. Several ion channels are regulated by a receptor localized to one part of the channel molecule which upon activation alters its shape. Neher and Sakmann have shown which parts of the molecule that constitute the “sensor” and the interior wall of the channel. They also showed how the channel regulates the passage of positively or negatively charged ions. This new knowledge and this new analytical tool has during the past ten years revolutionized modern biology, facilitated research, and contributed to the understanding of the cellular mechanisms underlying several diseases, including diabetes and cystic fibrosis.
Inside the cell membrane there is a well-defined environment, in which many complex biochemical processes take place. The interior of the cell differs in important respects from its outside. For example the contents of positive sodium and potassium ions and negatively charged chloride ions are quite different. This leads to a difference in electrical potential over the cell membrane, amounting to 0.03 to 0.1 volts. This is usually referred to as the membrane potential.
The cell uses the membrane potential in several ways. By rapidly opening channels for sodium ions the membrane potential is altered radically within a thousandth of a second. Cells in the nervous system communicate with each other by means of such electrical signals of around a tenth of a volt that rapidly travel along the nerve processes. When they reach the point of contact between two cells – the synapse – they induce the release of a transmitter substance. This substance affects receptors on the target cell, often by opening ion channels. The membrane potential is hereby altered so that the cell is stimulated or inhibited. The nervous system consists of a series of networks each comprised of nerve cells connected by synapses with different functions. New memory traces in the brain are for example created by altering the number of available ion channels in the synapses of a given network.
All cells function in a similar way. In fact, life itself begins with a change in membrane potential. As the sperm merges with the egg cell at the instant of fertilization ion channels are activated. The resultant change in membrane potential prevents the access of other sperm cells. All cells – for instance nerve cells, gland cells, and blood cells – have a characteristic set of ion channels that enable them to carry out their specific functions. The ion channels consist of single molecules or complexes of molecules, that forms the wall of the channel – or pore – that traverses the cell membrane and connects the exterior to the interior of the cell (Figure 1B and 1D). The diameter of the pore is so small that it corresponds to that of a single ion (0.5-0.6 millionths of a millimetre). An immediate change in the shape of the molecule leads to either an opening or a closure of the ion channel. This can occur upon activation of the receptor part of the molecule (Figure 1D) by a specific signal molecule. Alternatively a specific part of the molecule that senses changes in membrane potential can open or close the ion channel.
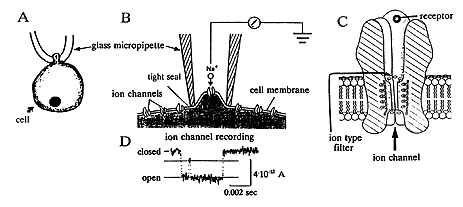 |
Figure 1. Registration of the flow of current through single ion channels using the recording technique of Neher and Sakmann. A schematically shows how a glass micropipette is brought in contact with the cell, and B, using a higher magnification, a part of the cell membrane, with ion channels, in close contact with the tip of the pipette. The interior of the pipette is connected to an electronic amplifier. C shows a channel in greater magnification with its receptor facing the exterior of the cell and its ion filter. D shows the current passing through the ion channel as it opens.
It has long been known that there is a rapid ion exchange over the cell membrane, but Neher and Sakmann were the first to show that specific ion channels actually exist. To elucidate how an ion channel operates it is necessary to be able to record how the channel opens and closes. This appeared elusive since the ionic current through a single ion channel is extraordinarily small. In addition, the small ion channel molecules are embedded in the cell membrane. Neher and Sakmann succeeded in solving these difficulties. They developed a thin glass micropipette (a thousandths of a millimeter in diameter) as a recording electrode. When it is brought in contact with the cell membrane, it will form a tight seal with the periphery of the pipette orifice (Figure 1A, B). As a consequence the exchange of ions between the inside of the pipette and the outside can only occur through the ion channel in the membrane fragment (Figure 1B). When a single ion channel opens, ions will move through the channel as an electric current, since they are charged. Through a refinement of the electronic equipment and the experimental conditions they succeeded in measuring this “microscopical” current by laborious methodological developments during the seventies (Figure 1C).
Ion channels are of different types. Some only permit the flow of positively charged sodium, potassium or calcium ions, others only negatively charged chloride ions. Neher and Sakmann discovered how this specificity is accomplished. One reason is the diameter of the ion channel, which is adapted to the diameter of a particular ion. In one class of ion channels, there are also two rings of positively or negatively charged amino acids. They form an ionic filter (see Figure 1D), which only permits ions with an opposite charge to pass through the filter. In particular Sakmann through a creative interaction with different molecular biologists elucidated how the different parts of the ion channel molecule(s) operate. Neher and Sakmann’s scientific achievements have radically changed our views on the function of the cell and the contents of text books of cell biology. Their methods are now used by thousands of scientists all over the world.
Nerve cells, as well as hormone-producing cells and cells engaged in the host defence (like mast cells) secrete different agents. They are stored in vesicles enclosed by a membrane. When the cell is stimulated the vesicles move to the cell surface. The cell and vesicle membranes fuse and the agent is liberated. The mast cell secretes histamine and other agents that give rise to local inflammatory reactions. The cells of the adrenal medulla liberate the stress hormone adrenaline, and the beta cells in the pancreas insulin. Neher elucidated the secretory processes in these cell types through the development of a new technique which records the fusion of the vesicle(s) with the cell membrane. Neher realized that the electric properties of a cell would change if its surface area increased making it possible to record the actual secretory process. Through further developments of their sophisticated equipment the resolution finally permitted recording of each little vesicle fusing with the cell membrane.
Neher and Sakmann also used the electrode pipette to inject different agents into the cell, and they could thereby investigate the different steps in the secretory process within the cell itself (see above). In this way a number of cellular secretory mechanisms have been clarified such as the role of cyclic AMP (see Nobel Prize to Sutherland 1971) or calcium ions. For instance, we now have a better understanding of how the hormone levels in the blood are maintained at a certain level.
Also the basal mechanisms underlying the secretion of insulin have been identified. The level of blood glucose controls the level of glucose within the insulin-forming cell, which in turn regulates the level of the energy rich substance ATP. ATP acts directly on a particular type of ion channel which controls the electric membrane potential of the cell. The change of membrane potential then indirectly influences other ion channels, which permit calcium ions to pass into the cell. The calcium ions subsequently trigger the insulin secretion. In diabetes the insulin secretion is out of order. Certain drugs commonly used to stimulate insulin secretion in diabetes act directly on the ATP-controlled ion channels.
Many other diseases depend entirely, or partially, on a defect regulation of ion channels, and a number of drugs act directly on ion channels. Many pathological mechanisms have been clarified during the eighties through ion channel studies, for instance cystic fibrosis (cloride ion channels), epilepsy (sodium and potassium ion channels), several cardio-vascular diseases (calcium ion channels), and neuro-muscular disorders like Lambert-Eatons disease (calcium ion channels). With the help of the technique of Neher and Sakmann it is now possible to tailormake drugs, to achieve an optimal effect on particular ion channels of importance in a given disease. Drugs against anxiety act for instance on certain inhibitory ionic channels in the brain. Alcohol, nicotine and other poisons act on yet other sets of ion channels.
In summary, Neher and Sakmann’s contributions have meant a revolution for the field of cell biology, for the understanding of different disease mechanisms, and opened a way to develop new and more specific drugs.
References
Alberts et al.: The Molecular Biology of the Cell. Garland Press, 1990, 2nd edition, pp. 156, 312-326, 1065-1084.
Grillner, S. I: N. Calder (ed.). Scientific Europe. Foundation Scientific Europe, 1990.
Grillner, S. & Hökfelt, T.: Svindlande snabb utveckling präglar neurovetenskapen. Läkartidningen 1990, 87, 2777-2786.
Rorsman, P. & Fredholm, B.B.: Jonkanaler – molekylär bakgrund till nervtransmission. Läkartidningen 1991, 88, 2868-2877.
